Publication List:
(68) Hu, J.; Su, S.; Zhang, H.; Gao, Y.; Yang, X.; Chen, Y.; Gong, H. "Nickel-catalysed stereoselective α-vinylation and arylation of peptide electrophiles", ChemRxiv, 2024, DOI: .
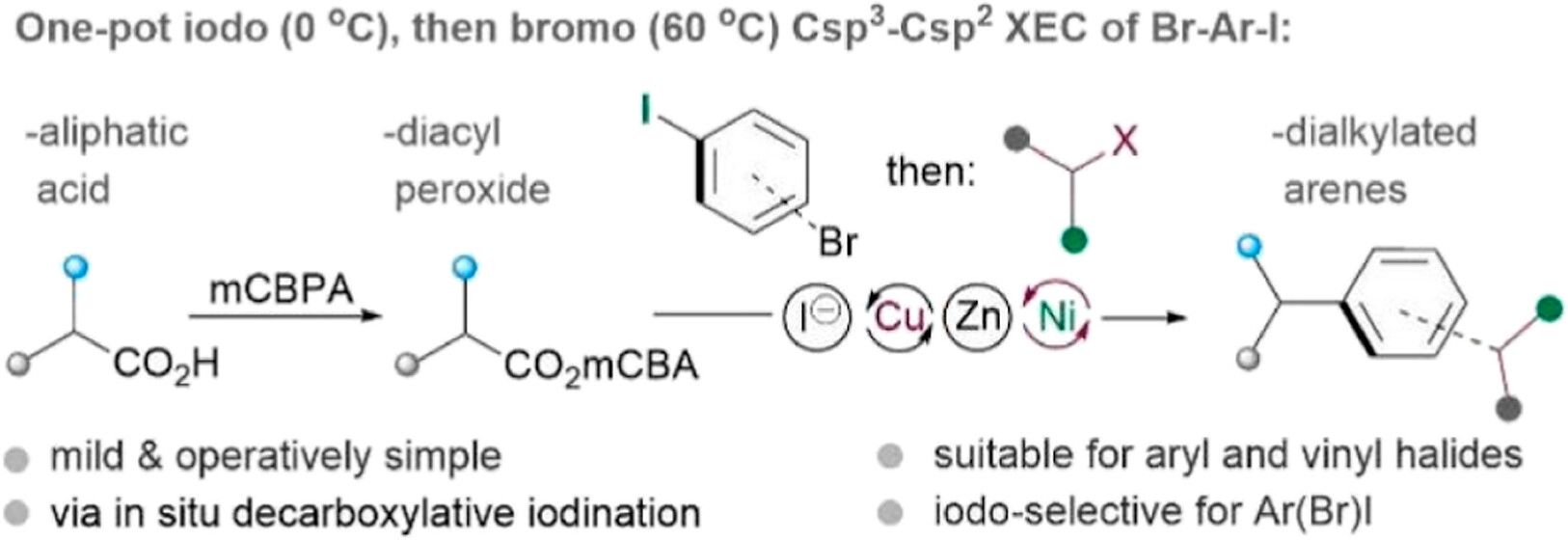
(67) Qiu, C.; Chen, Q.; Gong, H.* "Nickel-Catalyzed Reductive Decarboxylative Coupling of Diacyl Peroxides with Aryl/Vinyl Halides", Acs. Catal., 2024, 14, 12192−12198
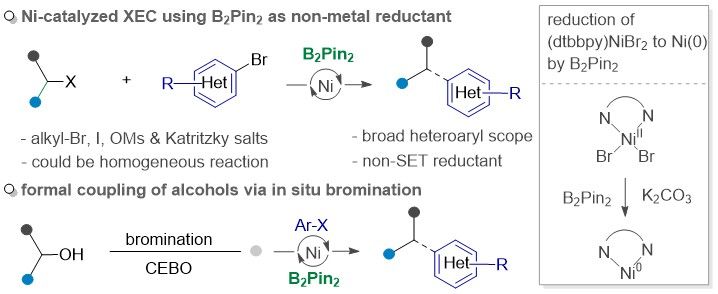
(66) Sun, D.; Gong, Y.; Chen, Y.;* Gong, H.* "Bis(pinacolato)diboron-Enabled Ni-Catalyzed Reductive Arylation/Vinylation of Alkyl Electrophiles", Adv. Sci., 2024, 2404301, DOI:10.1002/advs.202404301
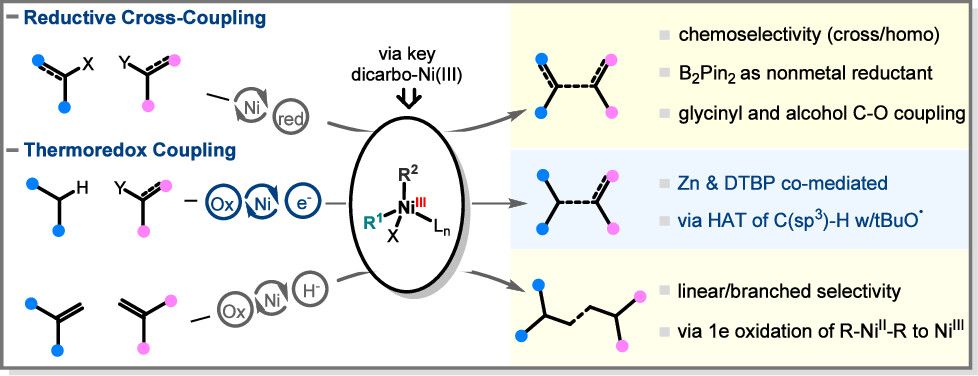
(65) Gong, Y.; Hu, J.; Qiu, C.; Gong, H.* "Insights into Recent Nickel-Catalyzed Reductive and Redox C–C Coupling of Electrophiles, C(sp3)–H Bonds and Alkenes", Acc. Chem. Res., 2024, 57, 8, 1149–1162
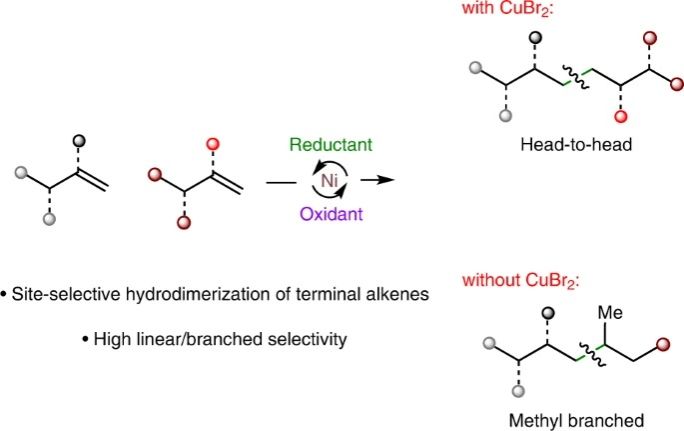
(64) Cheng, L.;‡ Liu, J.;‡ Chen, Y.; Gong, H.* "Nickel-catalysed hydrodimerization of unactivated terminal alkenes", Nature Synthesis, 2023, 2, 364–372 (‡These authors contributed equally.)
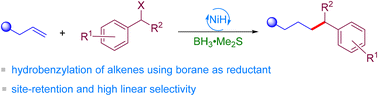
(63) Cheng, L.;‡ Lin, Q.;‡ Song, Y.; Chen, H.; Gong, H.*; Chen, Y.* "Ni-catalyzed regioselective hydrobenzylation of alkenes to afford C(sp3)-C(sp3) bonds using BH3 as a reductant", Org. Chem. Front. 2023, 10, 369–374 (‡These authors contributed equally.)
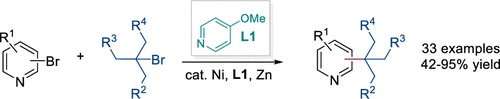
(62) Lin, Q.; Gong, H.*; Wu, F.* "Ni-Catalyzed Reductive Coupling of Heteroaryl Bromides with Tertiary Alkyl Halides", Org. Lett. 2022, 24, 8996–9000
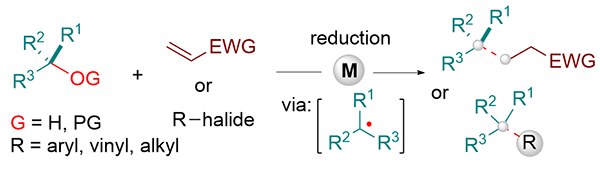
(61) Cheng, L.; Lin, Q.; Chen, Y.*; Gong, H.* "Recent Progress on Transition-Metal-Mediated Reductive C(sp3)-O Bond Radical Addition and Coupling Reactions", Synthesis 2022, 54, 4426–4446
(60) Gong, Y.; Su, L.; Zhu, Z.; Ye, Y.; Gong, H.* "Nickel-Catalyzed Thermal Redox Functionalization of C(sp3)−H Bonds with Carbon Electrophiles", Angew. Chem. Int. Ed. 2022, 61, DOI: 10.1002/anie.202201662 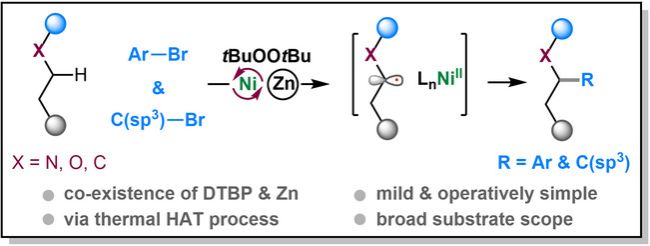
(59) Lin, Q.; Ma, G.;* Gong, H.* "Ni-catalyzed Formal Cross-Electrophile Coupling of Alcohols with Aryl Halides", Acs. Catal. 2021, 11, 14102–14109
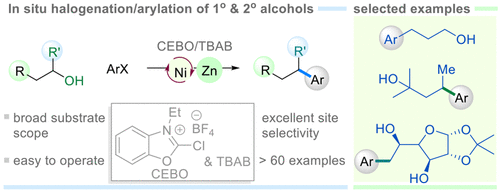
(58) Sun, D.; Ma, G.; Lei, C.;* Gong, H.* "Nickel-Catalyzed Asymmetric Reductive Arylation of α-Chlorosulfones with Aryl Halides", Chem. Sci. 2021, 12, 5253–5258 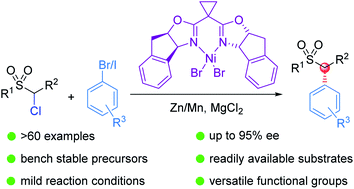
(57) Min, Y.; Ma, G.; Wang, X.;* Gong, H. "Nickel-Catalyzed Asymmetric Reductive Trifluormethylation of α-Bromotrifluoromethyl Alkanes with Aryl Halides", Angew. Chem. Int. Ed. 2021, 60, 9947–9952
(56) Su, L.; Ma, G.;* Song, Y.; Gong, H.* "Nickel-Catalyzed Reductive Synthesis of Aryl Thioesters", Org. Lett. 2021, 23, 2493–2497
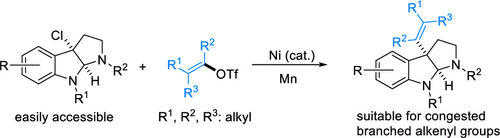
(55) Wang, J.; Gong, Y.; Sun, D.;* Gong, H.* "Nickel-catalyzed reductive benzylation of tertiary alkyl halides with benzyl chlorides and chloroformates", Org. Chem. Front. 2021, 8, 2944–2948
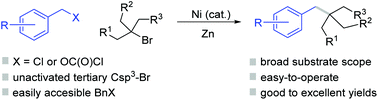
(54) Sun, Y.; Su, L.; Tong W.; Yao K.;* Gong H.* "Ni-Catalyzed Reductive Carbonylation of Alkyl Halides to Form Dialkyl Ketones Using Diphenyl Oxalate as CO Surrogate", Synlett 2021, 32, 1762–1766

(53) Ye, Y.; Ma, G.; Yao, K.; Gong, H.* "Zn-Mediated Hydrodeoxygenation of Tertiary Alkyl Oxalates", Synlett 2021, 32, 1625–1628 (Cluster: Modern Ni-Catalyzed Reactions) 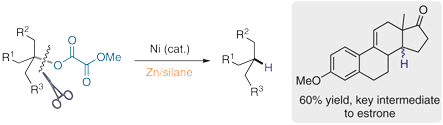
(52) Sha, Y.; Liu, J.; Wang, L.; Liang, D.; Wu, D.; Gong, H.* "Nickel-catalyzed reductive 1,3-diene formation from the cross-coupling of vinyl bromides", Org. Biomol. Chem. 2021, 19, 4887–4890
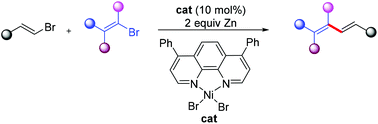
(51) Ye, Y.; Gong, H.* "Chromium catalyzed reductive chemoselective cross-coupling between anisole derivatives and aryl ester", Chin. J. Org. Chem. 2021, 40, 2588–2589 (50) Liu, J.; Gong, H.;* Zhu, S.* "BH3·Me2S: An Alternative Hydride Source for NiH-Catalyzed Reductive Migratory Hydroarylation and Hydroalkenylation of Alkenes", Eur. J. Org. Chem. 2021, 10, 1543–1546
(49) Zhu, Z.; Gong, Y.; Tong, W.; Wei C.;* Gong, H.* "Nickel-Catalyzed Reductive Synthesis of Aryl Thioesters", Org. Lett. 2021, 23, 2158–2163
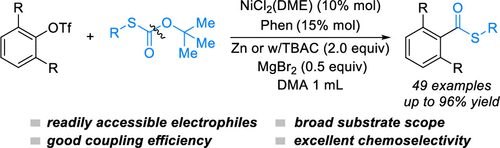
(48) Gong, Y.; Zhu, Z.; Qian, Q.; Tong, W.; Gong, H.* "Zn- and Cu-Catalyzed Coupling of Tertiary Alkyl Bromides and Oxalates to Forge Challenging C–O, C–S, and C–N Bonds", Org. Lett. 2021, 23, 1005–1010
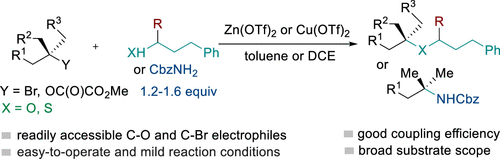
(47) Xue, W.;* Jia, X.; Wang, X.; Yin, Z.;* Gong, H.* "Nickel-catalyzed all-carbon quaternary centers formation with tertiary alkyl electrophiles", Chem. Soc. Rev. 2021, 50, 4162–4184
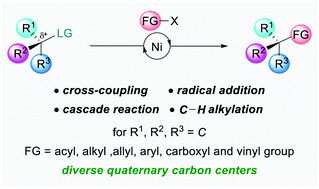
(46) Liu, J.; Gong, H.;* Zhu, S.* "Nickel-Catalyzed, Regio- and Enantioselective Benzylic Vinylation of Olefins with Vinyl Bromide", Angew. Chem. Int. Ed. 2021, 59, 4106–4110
(45) Tao, X.; Chen, Y.; Guo, J.; Wang, X.;* Gong, H.* "Preparation of α-Amino Acids via Ni-Catalyzed Reductive Vinylation and Arylation of α-Pivaloyloxy Glycine", Chem. Sci. 2021, 12, 220–226 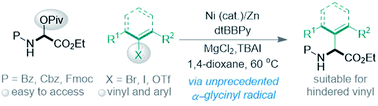
(44) Liu, J.; Ye, Y.; Sessler, J. L.; Gong, H.* "Cross-electrophile Coupling of Activated and Sterically Hindered Alkyl Halides and C-O bonds", Acc. Chem. Res. 2020, 53, 1833–1845
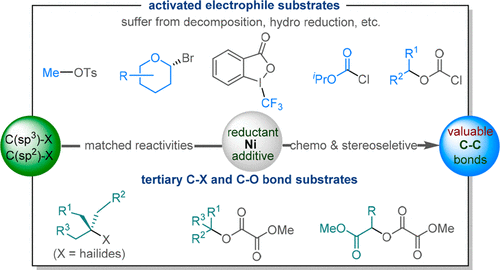
(43) Ma, G.; Chen, C.; Talukdar, S.; Zhao, X.; Lei, C.;* Gong, H.* "Metal Catalyst-Free Photo-Induced Alkyl C-O Bond Borylation of Tertiary Alkyl Oxalates", Chem. Commun. 2020, 57, 10219–10222 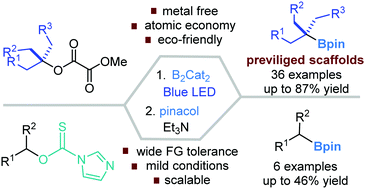
(42) Ye, Y.; Chen, H.; Ma, G.; Gong, H.* "Iron-Catalyzed Reductive Vinylation of Tertiary Alkyl Oxalates with Vinyl Bromides", Org. Lett. 2020, 22, 2070–2075
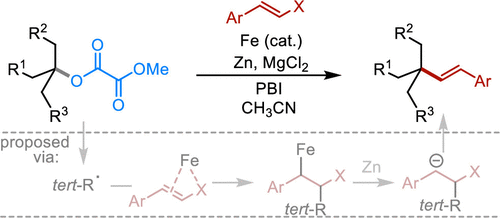
(41) Chen, H.; Ye, Y.; Tong, W.; Fang, J.; Gong, H.* "Formation of Allylated Quaternary Carbon Centers via C–O/C–O Bond Fragmentation of Oxalates and Allyl Carbonates", Chem. Commun. 2020, 56, 454–457 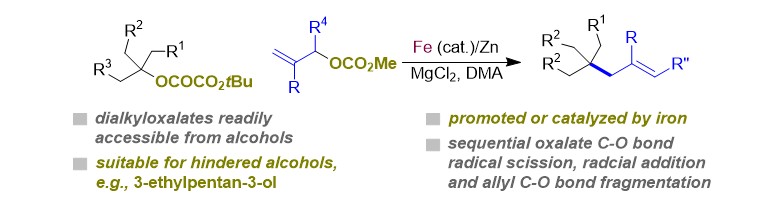
(40) Sun, Y.; Gu, J.; Wang, H.; Sessler, J. L.;* Thordason, P.;* Lin, Y.-J.; Gong, H.* "AAAA-DDDD Quadruple H-Bond-Assisted Ionic Interactions: Robust Bis(Guanidinium)/DiCarboxylate Heteroduplexes in Water", J. Am. Chem. Soc. 2019, 141, 20146–20154 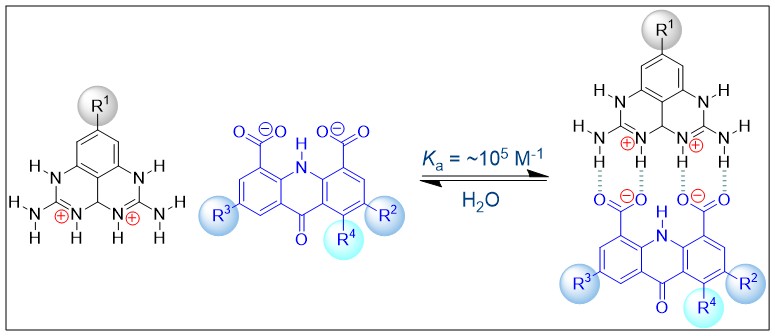
(39) Liu, J.; Lei, C.; Gong, H.* "Nickel-Catalyzed Reductive Coupling of Glucosyl Halides with Aryl/Vinyl Halides Enabling β-Selective Preparation of C-Aryl/Vinyl Glucosides", Sci. Chin. Chem. 2019, 62, 1492–1496 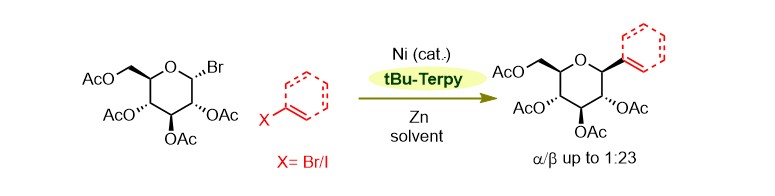
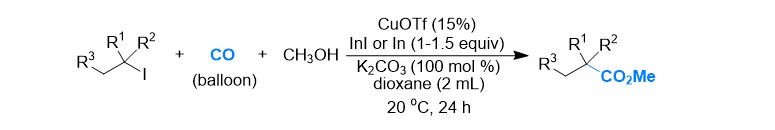
(38) Chen, Y.; Su, L.; Gong, H.* "Cu-Catalyzed and In-Mediated Ester Formation via Carbonylative Coupling of Alkyl Iodides under Balloom CO", Org. Lett. 2019, 21, 4689–4693
(37) Gao, M.; Sun, D.; Gong, H.* "Ni-Catalyzed Reductive C–O Bond Arylation of Oxalates Derived from α-Hydroxy Esters with Aryl Halides", Org. Lett. 2019, 21, 1645–1648 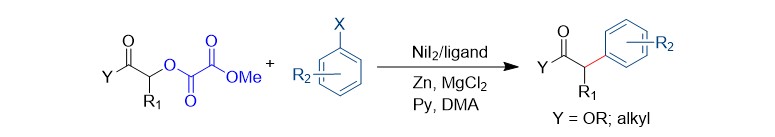
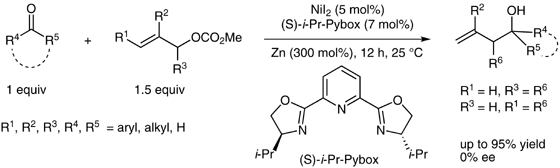
(36) Ye, Y.; Chen, H.; Sessler, J. L.; Gong, H.* "Zn-Mediated Fragmentation of Tertiary Alkyl Oxalates Enabling Formation of Alkylated and Arylated Quaternary Carbon Centers", J. Am. Chem. Soc. 2019, 141, 820–824 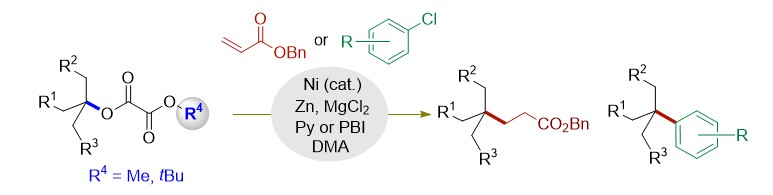
(35) Luo, L.; Zhai, X.-Y.; Wang, Y.-W.; Peng, Y.;* Gong, H.* "Divergent Total Syntheses of C3a–C7’ Linked Diketopiperazine Alkaloids (+)-Asperazine and (+)-Pestalazine A Enabled by a Ni-Catalyzed Reductive Coupling of Tertiary Alkyl Chloride", Chem. – Eur. J. 2019, 25, 989–992
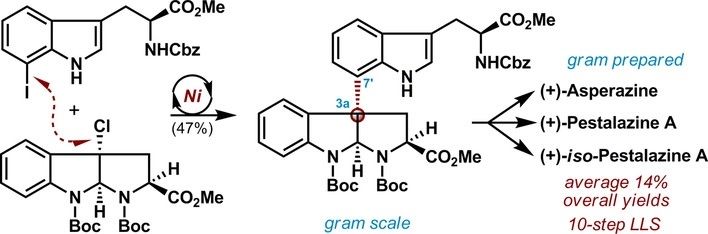
(34) Liu, J. Gong, H.* "Stereoselective Preparation of α‑C‑Vinyl/Aryl Glycosides via Nickel Catalyzed Reductive Coupling of Glycosyl Halides with Vinyl and Aryl Halides", Org. Lett. 2018, 20, 7991–7995 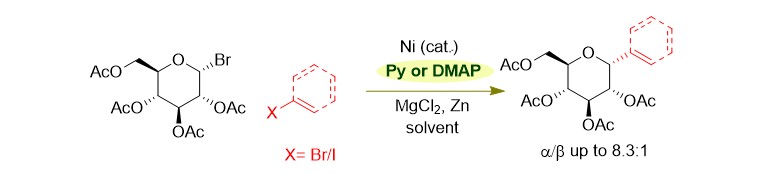
(33) Wang, X.; Ma, G.; Peng, Y; Pitsch, C. E.; Moll, B. J.; Ly, T. D.; Wang, X.;* Gong, H.* "Ni-Catalyzed Reductive Coupling of Electron-Rich Aryl Iodides with Tertiary Alkyl Halides", J. Am. Chem. Soc. 2018, 140, 14490–14497 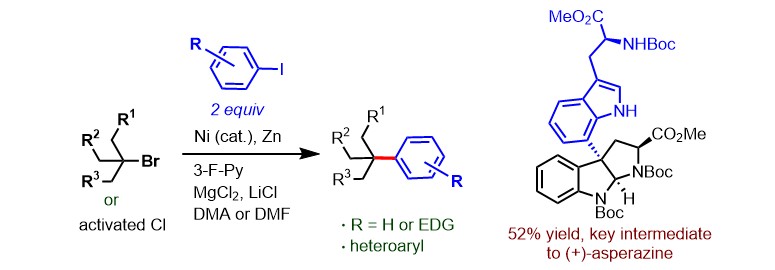
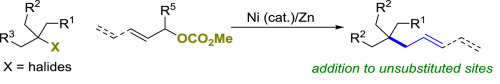
(32) Yu, Y.; Chen, H.; Qian, Q.; Yao, K.; Gong, H. "An extension of nickel-catalyzed reductive coupling between tertiary alkyl halides with allylic carbonates", Tetrahedron, 2018, 74, 5651–5658
(31) Chen, Y.; Ma, G.;* Gong, H.* "Copper-Catalyzed Reductive Trifluoromethylation of Alkyl Iodides with Togni's Reagent", Org. Lett. 2018, 20, 4677–4680 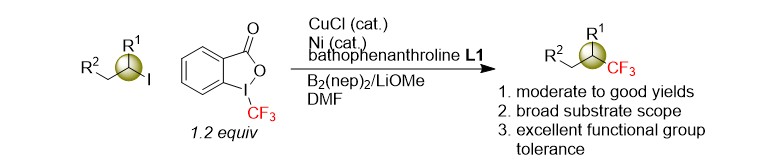
(30) Chen, H.; Jia, X.; Yu, Y.; Qian, Q.; Gong, H.* "Nickel-Catalyzed Reductive Allylation of Tertiary Alkyl Halides with Allylic Carbonates", Angew. Chem., Chem. Int. 2017, 56, 13103–13106 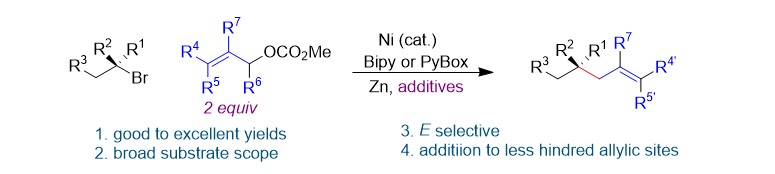
(29) Wang, J.; Zhao, J.; Gong, H.* "Nickel-catalyzed methylation of aryl halides/tosylates with methyl tosylate", Chem. Commun. 2017, 53, 10180–10183 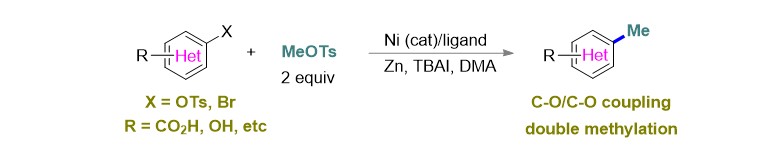
(28) Gu, J.; Qiu, C.; Lu, W.; Qian, Q.; Lin, K.; Gong, H.* "Nickel-Catalyzed Reductive Cross-Coupling of Vinyl Bromides with Unactivated Alkyl Halides", Synthesis 2017, 49, 1867–1873
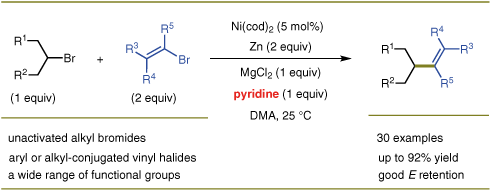
(27) Zheng, M.; Xue, W.; Xue, T.; Gong, H.* "Ester Formation via Nickel-Catalyzed Reductive Coupling of Alkyl Halides with Chloroformates", Org. Lett. 2016, 18, 6152–6155 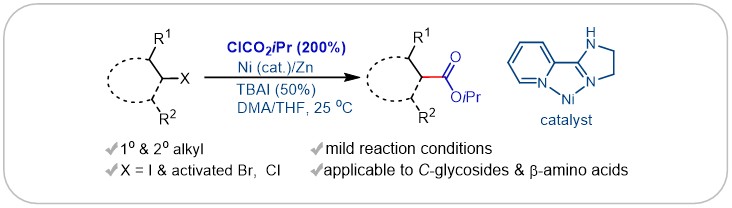
(26) Liu, J.; Ren, Q.; Zhang, Q.; Gong, H.* "Preparation of Vinyl Arenes by Nickel-Catalyzed Reductive Coupling of Aryl Halides with Vinyl Bromides", Angew. Chem., Chem. Int. 2016, 55, 15544–15548 
(25) Qiu, C.; Yao, K.; Zhang, X. Gong, H.* "Ni-Catalyzed Reductive Coupling of α-Halocarbonyl Derivatives with Vinyl Bromide", Org. Biomol. Chem. 2016, 14, 11332–11335
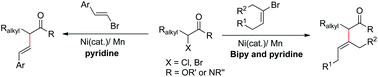
(24) Zhang, Q.; Wang, X. Qian, Q.; Gong, H.* "Nickel-Catalyzed Reductive Benzylation of Aryl Halides", Synthesis 2016, 48, 2829–2836 (Invited for special topics of Ni and Fe-catalyzed reactions)
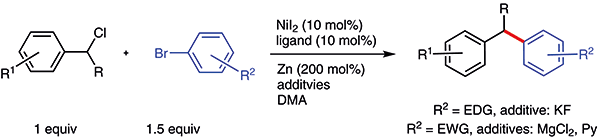
(23) Wang, X.; Dai, Y.; Gong, H.* "Nickel-Catalyzed Reductive Couplings", Top. Curr. Chem. 2016, 374, 43 (Invited review in a chapter of Ni and Fe-catalyzed reactions) (22) Wang, B.; Dai, Y.;* Tong, W.* Gong, H.* "Ni-Catalyzed Reductive Addition of Alkyl Halides to Isocyanides", Org. Biomol. Chem. 2015, 13, 11418–11421
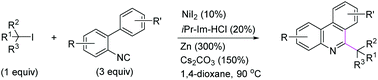
(21) Wang, X.; Wang, S.; Xue, W.; Gong, H.* "Nickel-Catalyzed Reductive Coupling of Aryl Halides with Unactivated tert-Alkyl Halides", J. Am. Chem. Soc. 2015, 137, 11562–11565 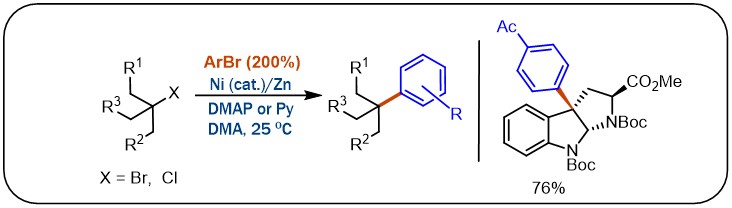
(20) Gu, J.; Wang, X.; Xue, W. Gong, H.* "Nickel-Catalyzed Reductive Coupling of Alkyl Halides with Other Electrophiles: Concept and Mechanistic Considerations", Org. Chem. Front. 2015, 3, 1411–1421 (Invited Tutorial Account) 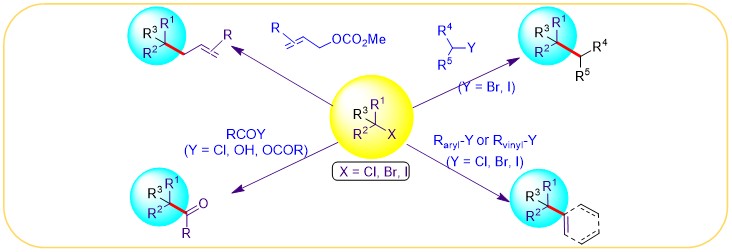
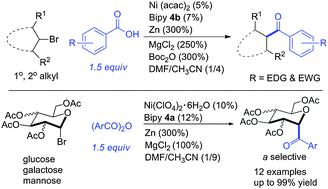
(19) Jia, X.; Zhang, X.; Qian, Q.; Gong, H.* "Nickel-Catalyzed Reductive Coupling of Alkyl and Glycosyl Halides with Aryl Acids and Anhydrides", Chem. Commun. 2015, 51, 10302–10305
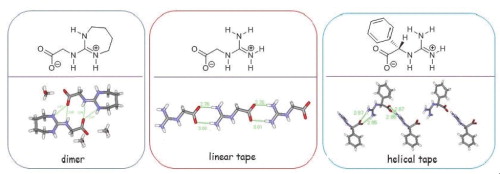
(18) Wang, W.; Gu, J.; Tong, W.; Gong, H.* "Solid State Studies of the Diionic Compounds based on Guanidine/Carboxyaltes", Tetrahedron Lett. 2015, 56, 2684–2687
(17) Zhao, C.; Jia, X.; Wang, X.; Gong, H.* "Ni-Catalyzed Reductive Coupling of Alkyl Acids with Unactivated tertiary Alkyl and Glycosyl Halides", J. Am. Chem. Soc. 2014, 136, 17645–17651 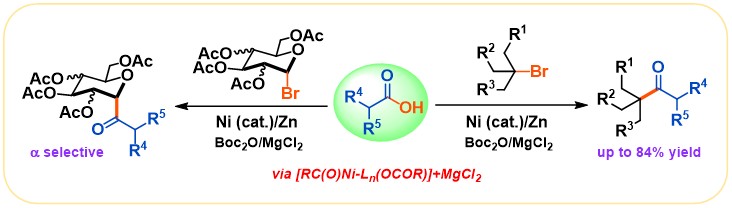
(16) Liang, Z.; Xue, W.; Lin, K.; Gong, H.* "Nickel-Catalyzed Reductive Methylation of Alkyl Halides and Acid Chlorides with Methyl p-Tosylate", Org. Lett. 2014, 16, 5620–5623
(15) Ren, Q*, Jiang, F.; Gong, H. "DFT Study of the Single Electron Transfer Mechanisms in Ni-Catalzyed Reductive Cross-Coupling of Aryl Bromides and Alkyl Bromides", J. Organomet. Chem. 2014, 770, 130–135
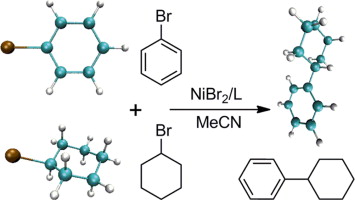
(14) Xue, W.; Xu, H.; Liang, Z.; Qian, Q.; Gong, H.* "Ni-Catalyzed Reductive Cyclization of Alkyl Dihalides", Org. Lett. 2014, 16, 4984-4987 (Highlighted in Synfacts, 2015, 11(1), 0076) 
(13) Zhao, C.; Tan, Z.; Liang, Z.; Deng, W.; Gong, H.* "Ni-Catalyzed Reductive Allylation of Ketones with Allylic Carbonates", Synthesis 2014, 46, 1901–1907 (invited for a special topics issue on nickel chemistry)
(12) Tan, Z.; Wan, X.; Zang, Z.; Qian, Q.; Deng, W.; Gong, H.* "Ni-Catalyzed Asymmetric Reductive Allylation of Aldehydes with Allylic Carbonates", Chem. Commun. 2014, 50, 3827 (Highlighted in Synfacts 2014, 10(7), 0725-By Hisashi Yamamoto at Univ of Chicago) 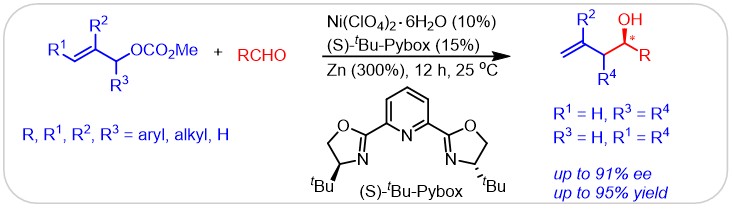
(11) Xu, H.; Zhao, C.; Qian, Q.; Deng, W.; Gong, H.* "Nickel-catalyzed cross-coupling of unactivated alkyl halides using bis(pinacolato)diboron as reductant", Chem. Sci. 2013, 4, 4022–4029 (Highlighted as Synstories in Synform, 2013; 9(12): A145-A156) 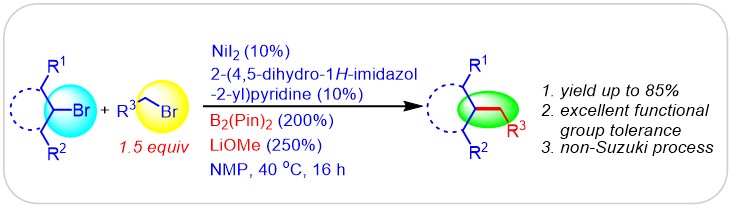
(10) Lu, W.; Liang, Z.; Zhang, Y.; Wu, F.; Qian, Q.; Gong, H.* "Gram-Scale Ketone Synthesis by Direct Reductive Coupling of Alkyl Iodides with Acid Chlorides", Synthesis, 2013, 45, 2234–2240. Invited PSP (Practical Synthetic Procedures)

(9) Qian, Q.; Zang, Z.; Chen, Y.; Tong, W.; Gong, H.* “Nickel- and Cobalt-Catalyzed Reactions of Alkyl Halides with Alkenes", Mini-Rev. Med. Chem. 2013, 13, 802–813 (Invited review) (8) Qian, Q.;* Zang, Z.; Chen, Y.; Lin, K.;* Gong, H. "Nickel-Catalyzed Reductive Coupling of Aryl Halides", Synlett, 2013, 24, 619–624
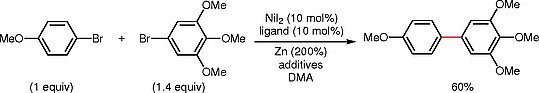
(7) Cui, X.; Wang, S.; Zhang, Y.; Qian, Q.;* Gong, H.* “Nickel-Catalyzed Reductive Allylation of Aryl Bromides with Allylic Acetate", Org. Biomol. Chem. 2013, 11, 3094–3097

(6) Wang, S.; Qian, Q.; Gong H.* "Nickel-Catalyzed Reductive Coupling of Aryl Halides with Secondary Alkyl Bromides and Allylic Acetate", Org. Lett. 2012, 14, 3352–3355 (Highlighted in Synfacts 2012, 8(10), 1130 and in Organic-chemistry.org) 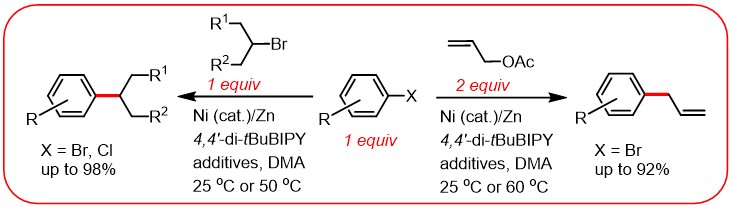
(5) Yin, H.; Zhao, C.; You, H.; Lin, K.; Gong, H.* "Mild Ketone Formation via Ni-Catalyzed Reductive Coupling of Unactivated Alkyl Halides with Acid Anhydrides", Chem. Commun. 2012, 48, 7034–7036 (Highlighted in Synfacts 2012, 8(10), 1128) 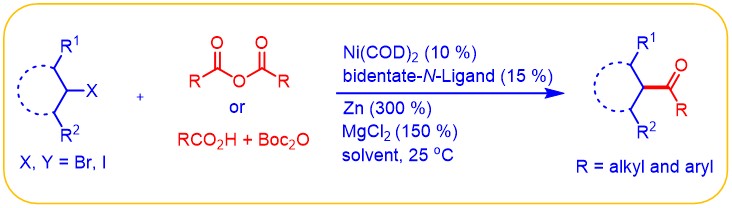
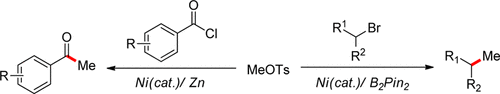
(4) Wu, F.; Lu, W.; Qian, Q.; Ren, Q.; Gong, H.* "Ketone Formation via Mild Nickel-Catalyzed Reductive Coupling of Alkyl Halides with Aryl Acid Chlorides", Org. Lett. 2012, 14, 3044–3047 (Highlighted in Synfacts 2012, 8(10), 1129) 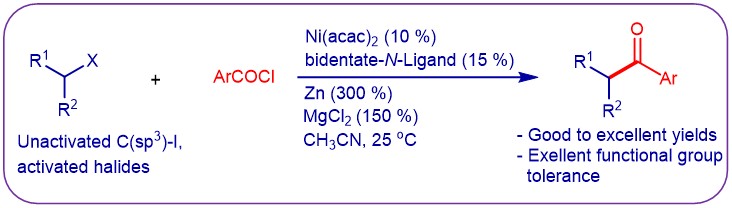
(3) Dai, Y.; Wu, F.; Zang, Z.; You, H.; Gong, H.* "Ni-Catalyzed Reductive Allylation of Unactivated Alkyl Halides with Allylic Carbonates", Chem. – Eur. J. 2012, 18, 808–812 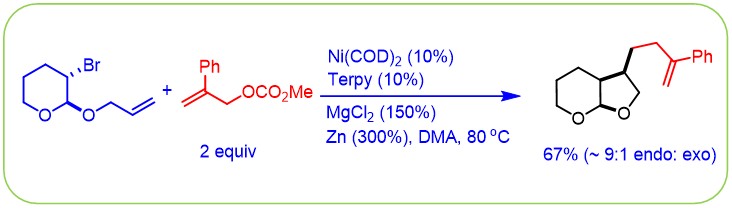
(2) Yu, X.; Yang, T.; Wang S.; Xu H.; Gong, H.* "Nickel-Catalyzed Reductive Cross-Coupling of Unactivated Alkyl Halides", Org. Lett. 2011, 13, 2138–2141 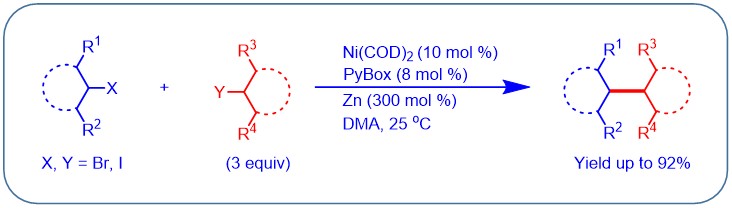
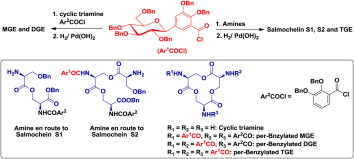
(1) Yu, X.; Dai, Y.; Yang, T.; Gagné, M. R.; Gong, H.* "Facile Synthesis of Salmochelin S1, S2, MGE, DGE and TGE", Tetrahedron 2011, 67, 144–151
|